Neutron activation analysis (NAA)
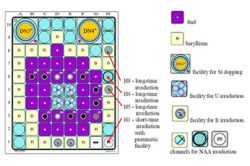
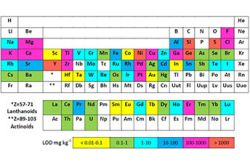
NAA is a primary method of measurement for quantitative multi-elemental analysis. Using irradiation in the LVR-15 reactor, where neutron fluence rates of up to 5·1013 cm-1 s-1 are available in vertical channels H1, H5, H6, H8 (cf. Fig. 1), detection limits range from μg kg-1 up to tens of percent, depending on the particular element and bulk matrix composition (cf. Fig. 2).
Using both short-time (10 s - 3 min.) and long-time (several hours - several days) irradiations, information about concentrations of up to 65 elements can be obtained, in many cases by non-destructive, so-called instrumental neutron activation analysis - INAA.
Short-time irradiation typically allows the concentration of the following elements to be determined:
F, Na, Mg, Al, Si, S, Cl, Ca, Ti, V, Mn, Ni, Cu, Y, Rh, In, I, Dy, U
Long-time irradiation enables determination of the following elements:
Na, K, Ca, Sc, Cr, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Rb, Sr, Zr, Nb, Mo, Ru, Pd, Ag, Cd, Sn, Sb, Te, Cs, Ba, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hga, Th, U
a - Hg losses on irradiation in polyethylene capsules possible
Advantages of NAA
- Minimal sample handling prior to irradiation leading to a minimal risk of contamination
- Possibility to perform analysis non-destructively, using INAA
- Large dynamic range of concentrations, which can be determined, from μg kg-1 to tens of percent
- Large dynamic range of sample masses, from a few mg to several hundred mg
- High trueness and low uncertainty of results, which are traceable to the SI unit of mass fraction, because NAA with relative standardization has recently been recognized as a primary ratio method
- Both relative and k0-NAA standardization available, the latter especially suitable for panoramic analyses
- NAA procedures with radiochemical separation (radiochemical neutron activation analysis - RNAA) available for determination of selected elements, namely V, Cr, Mn, Co, Ni, As, Se, Mo, Ag, Cd, Sb, I, rare earth elements, Re, Pt, Au, and Hg with LOD about two or three orders magnitude lower compared with those given in
Typical applications
- Environmental control and monitoring - analysis of aerosols, soils, sewage sludges, biomonitors
- Geo- and cosmochemistry - elemental characterization of rocks, minerals, tektites, meteorites
- Geomycology - elemental characterization of mushrooms and their substrates
- Nutritional science - determination of essential and toxic trace elements in foodstuffs and beverages
- Biomedicine - determination of essential and toxic trace elements in human and animal tissues
- Material science - elemental characterization of various material, e.g. high-tech materials that are difficult to analyze by other analytical methods
- Chemometry - quality control analyses to check performance of other analytical techniques, homogeneity testing and certification analyses of newly prepared reference materials
X-Ray fluorescence analysis (XRF)
X-ray fluorescence analysis is surface multi-elemental analysis technique with a very wide field of practical applications, especially those requiring non-destructive analytical method. With our modern X-ray fluorescence spectrometer Spectro Midex (Spectro Analytical Instruments, Germany) equipped with helium flush of the sample – detector space, we can determine a wide range of elements - from Mg to U. The motorized xyz sample stage is used for both area and linear scan with resolution from 0.1 mm to 2 mm. Detection limits depend on the sample preparation and are usually in the range of hundreds of μg kg-1 up to tens of per cent.
Advantages of XRF
- non-destructive analysis
- very fast qualitative analysis
- possibility to analyze sample “as is”
- possibility to determine low-Z elements like Al, Si, P, S
- Analysis of liquid samples (special calibrations and equipment is required)
Typical applications
- Archaeological samples – analysis of metal and glass artefacts
- Geological samples –semi-quantitative determination of elemental composition
- Alloy analysis – qualitative and quantitative analysis (needs proper calibration)
- Analyses concerning The Restriction of the use of certain Hazardous Substances in electrical and electronic equipment - RoHS (Directive 2002/95/EC)